Stroll through any supermarket, and you will see many sorts of packaging, bags, boxes, movies, and containers. The challenge of keeping ink movement from the printed data on food and staple packaging keeps numerous physicists, printers, and brand leaders awake at night.
It's easy to understand their nervousness. A few guidelines, like the Swiss Ordinance and the Nestlé Guidelines on Food Contact materials, require tough testing to guarantee contaminant levels measure under 10 parts for every billion.
For some perspective on how small an amount this is, consider that one pack of sugar in an Olympic-size swimming pool is a concentration of one part per billion. Now think about measuring for that.
To understand how food packaging stays below these contaminant thresholds, we must first understand what ink migration is, what causes it, and what options there are for preventing it.
What is Ink Migration in Consumer Products
Ink migration is the transfer or bleed-through of ink substances from the packaging material into consumer goods such as food, medicines or health and beauty products. What’s printed on the outside ends up inside.
Ink Migration is Not Good For Several Reasons:
- - It puts consumer health at risk
- - Changes the flavour and odour of products
- - It violates regulations
- - It can damage the brand reputation
Added together, that means trouble for the brand, their supply chain partners, and the consumers.
Intentionally Added Compounds
The components of inks–those intentionally added compounds–can be the most significant source of contamination. These include monomers, antioxidants, plasticizers, and photo initiators.
Non-Intentionally Added Substances
No less troublesome are the non-intentionally added substances (NIAS), which are difficult to detect and assess after being identified. The sources of NIAS vary.
Impurities in the raw materials of the ink might present non-deliberate substances. Another source might be the results of chemical reactions that could occur during packaging, ecological impacts during transportation or capacity. Purchasers might trigger responses that make perilous mixtures.
The Value of Low Migration Inks
The market for low-migration inks is strong, with pharmaceuticals accounting for 25% of product demand, according to a Global Market Insights, Inc. report. Total revenue could hit $480 million by 2025 compared to $300 million in 2018.
In North America, the market size for low-migration printing inks is projected to grow at over 7% CAGR, according to the market research report.
Types of Ink Migration
Set-off Migration
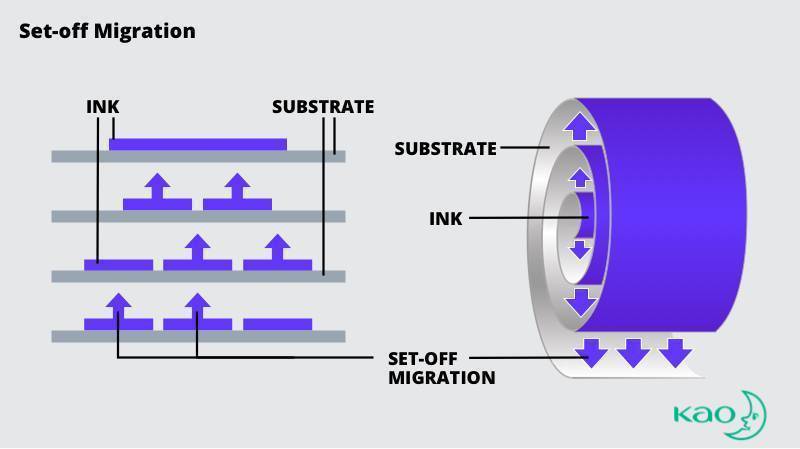
Ink migration is “set-off” when it transfers from the printed side of one package to the reverse side of another package.
When roll-printed packaging materials are re-wound, the ink may transfer to the reverse side. Similarly, stacking printed cups or other consumer products has the potential to transfer ink to the inside of the cup.
Diffusion Migration
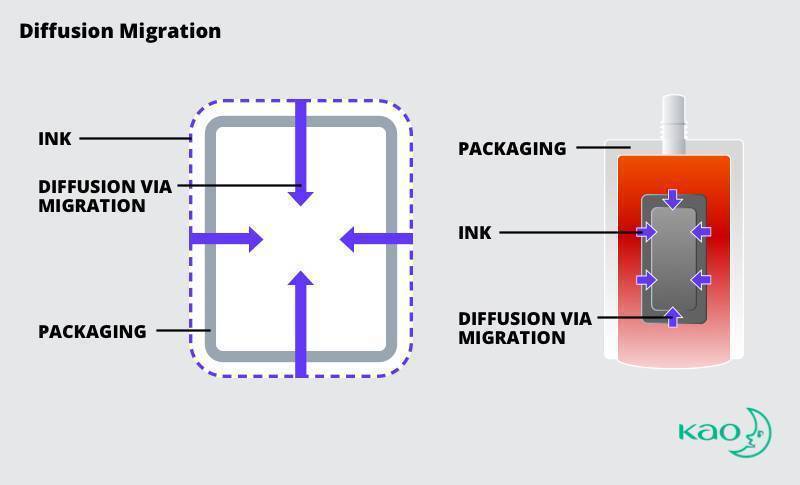
When ink migrates through diffusion, substances pass through the substrate from the printed side of the substrate to contact the food, medicine, or health and beauty product encased in the packaging. The substrate for the consumer product may not block the chemicals in ink. The ink and substrate may interact with environmental conditions to allow migration or introduction of NIAS.
Gas-Phase Migration
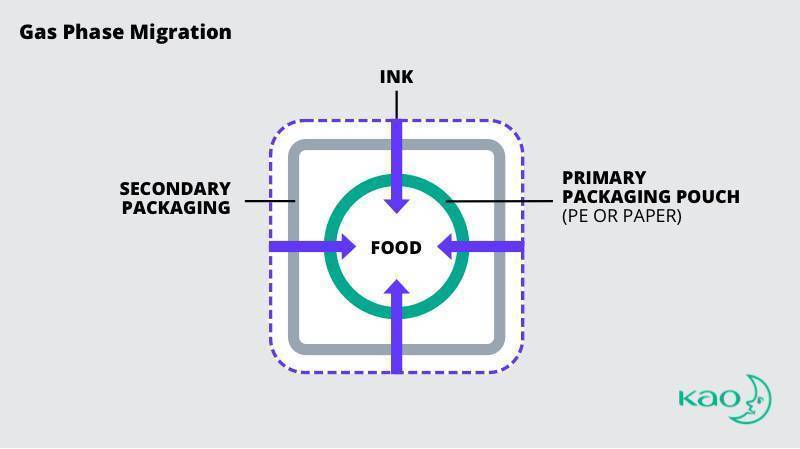
Gas-phase ink migration happens when volatile chemicals and compounds in the ink transfer to the interior product through evaporation, or from heating, baking, or boiling food products.
While the primary packaging of consumer products is the obvious source of gas-phase migration, academic studies have confirmed ink compounds used in secondary packaging have migrated through primary packaging.
Ink Migration Barriers
Glass and metal packaging are considered “absolute” barriers. Any ink can be used on the exterior of these packaging materials because these materials prevent migration. Other materials must be evaluated for the potential of ink migration. Both paper and plastic packaging pose a risk of migration.
What Causes Ink Migration or Contamination?
It may be called ink migration, but the ink is only one of the numerous complex factors that contribute to possible product contamination.
Kristin Adams, the marketing manager for Kao Collins, said, “The term low-migration actually defines the selection of materials as well as the ink. So regardless of how ink is formulated, the ink alone cannot be considered low-migration until it’s actually applied to a specific substrate.”
Packaging Materials
Consumers expect packaging materials to maintain freshness and keep products free of toxic contamination. That’s why testing must ensure the packaging materials and ink choice don’t negatively interact.
The Printing Environment
Contaminants from the equipment used for printing can introduce catalysts that affect either the ink or packaging material–or both. While the ink and packaging material have been verified to prevent dangerous migration, external factors may cause unintended reactions.
Incomplete Curing
Extensive and meticulous ink testing may prove an ink to be safe in ideal conditions. Risk is introduced if the testing conditions are not followed during production. Incomplete curing may introduce toxic materials that would not otherwise exist if the ink had completely dried.
Post-packaging Influences
Environmental conditions introduced during shipping and storage can’t be ignored. The packaging and inks must be tested to meet tolerances of extreme heat, cold, and humidity. In the hands of the consumer, the product must remain safe when boiled, frozen, or heated in a microwave or conventional oven.